Biological Processes and Interactions
Biochemical Reactions: Important Cellular Changes
MINDS ON
Some Key Terms
Look for these terms in this Activity. You should practice using them as you compose your responses to the upcoming tasks.
Realize, though, that this list is not exhaustive so you should look for opportunities to include other relevant terminology related to biochemical reactions.
|
enzyme |
reactant |
product |
|
hydrolysis |
dehydration synthesis |
phosphorylation |
|
reduction |
oxidation |
isomerization |
|
decarboxylation |
anabolism |
catabolism |
|
metabolism |
|
|
Changes
Before we begin looking at specific biological reactions, let’s take a step back and examine a change involving objects we can see with our eyes and hold with our hands. Suppose you are interested in purchasing a pizza store and want to know if it’s a good business decision. You want to investigate without the present owner knowing because you fear the owner will raise the price. So, instead of going into the store, watching what happens or asking to examine the books that record expenses and profits, you decide to watch the store from outside.
You observe how often trucks arrive with pizza dough, pizza toppings (cheese, pepperoni, etc.), and other supplies. You also observe how often pizzas leave the store, either with customers or with delivery people to deliver pizzas to customers.
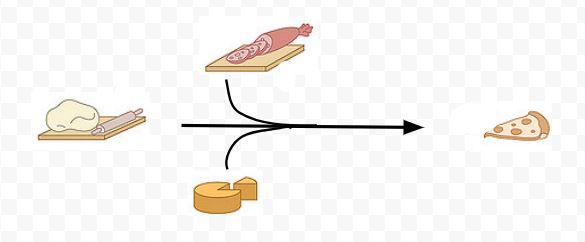
In this analogy, the pizza supplies are the reactants and the boxed pizzas that are delivered to customers are the end products. The workers within the store that shape the dough, add the toppings and place the pizzas in ovens and finally in boxes are the equivalent of the enzymes.
Although we don't actually see the workers doing their job, we can infer that if the store is using large quantities of reactants (dough and toppings) and/or making large numbers of end products (boxed pizzas) that the workers (enzymes) must be very active. Also, we can describe the change by identifying how different reactants (dough and toppings) are changed.
We could represent this change in different ways. We could show this overall change similar to a biochemical equation.
We can also describe this change using a flowchart. In this case we are describing individual changes in the order that they occur.
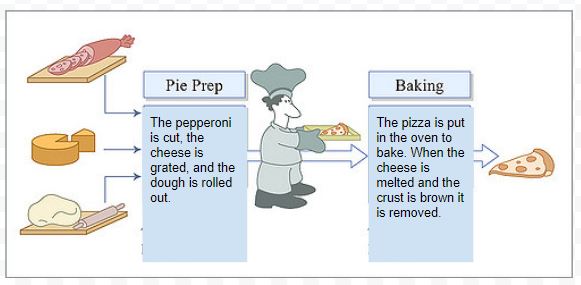
 How do small steps lead to change?
How do small steps lead to change?
Choose a change that is part of your everyday life. Find a picture or sketch a diagram to show this change like the examples outlined above. Describe the change as either a biochemical equation or a flowchart.
 How do small steps lead to changes?
How do small steps lead to changes?
Changes 1. Is the change that you identified a physical change or a chemical change? How can you tell?
ACTION
Energy in Reactions
In Biology there are several types of energy that are important to understand. As you learn more about Biology in future years you will learn about more types of energy. For now, these are the most common types of energy important to this course:
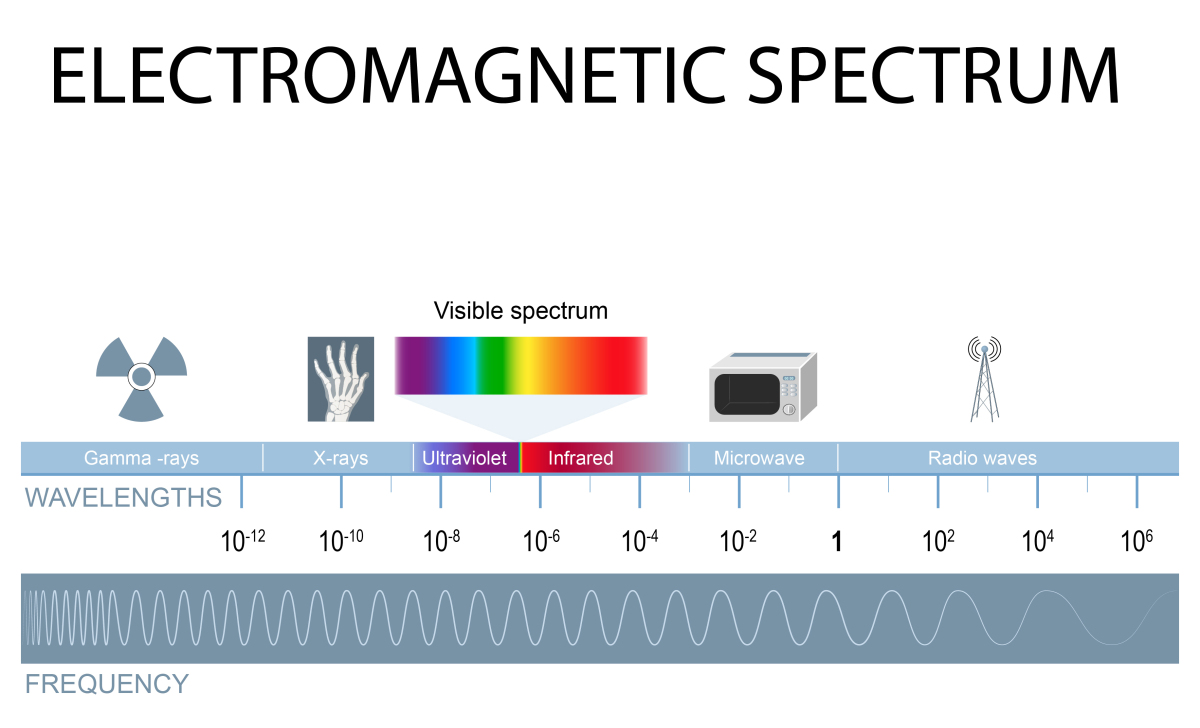
Light used by many autotrophs is a form of electromagnetic energy. You will remember that visible light is the middle portion of the electromagnetic spectrum.
Heat is also known as thermal energy.
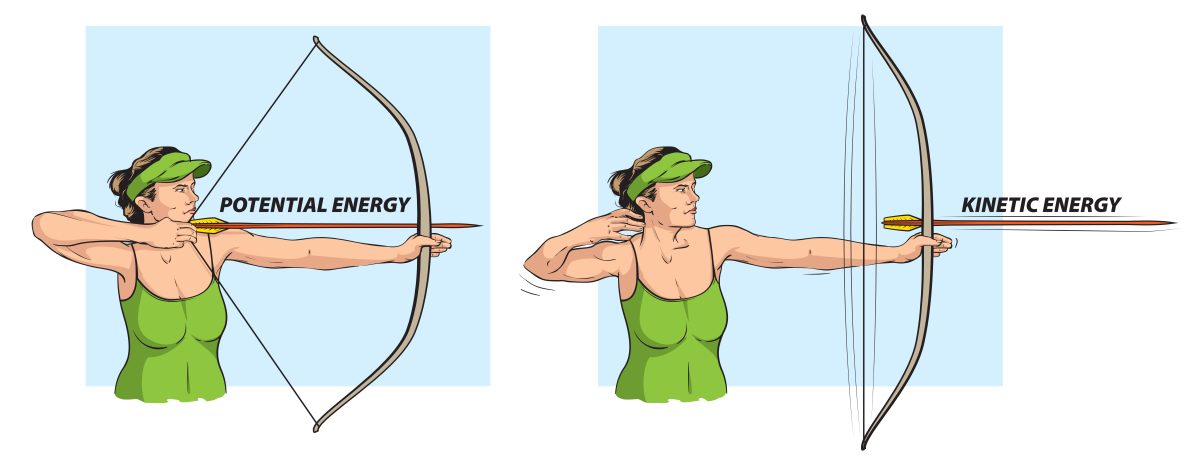
Potential energy is the energy stored in the bonds of biological molecules. For example, when we say that energy is transferred from a molecule of glucose to ATP, what is happening is that the potential energy is transferred from the bonds in glucose to the bonds in ATP.
Kinetic energy is the energy of a moving object. A good example of this is how ATP is used by a motor protein to move a vesicle (definition:a small vacuole typically found in animal cells) along a microtubule in a cell.
The reactions that occur in the body are collectively known as metabolism. (definition:the sum of the chemical reactions that take place within each cell of a living organism, and that provide energy for vital processes and for synthesizing new organic material.) In everyday life we also use the term metabolism to describe how quickly a person can burn food. In this Activity we are looking at the types of reactions, not the speed of those reactions. That being said, all metabolic reactions involve changes of energy.
 How does understanding change?
How does understanding change?
Understanding 20. Fast metabolism, or slow metabolism. Describe what you understand these terms to mean. Since there is no “correct” answer to this question, is there a specific example from everyday life that you’re basing your answer on?
Metabolism can be further divided into two groups:
- anabolic - a reaction that makes a larger molecule from smaller ones reactions and
- catabolic - a reaction that makes smaller molecules from a larger one.
Watch either one of these video to identify differences and similarities between anabolism and catabolism. You will use this information in the next section.
Interestingly, one common biochemical reaction is neither anabolism nor catabolism: isomerization.
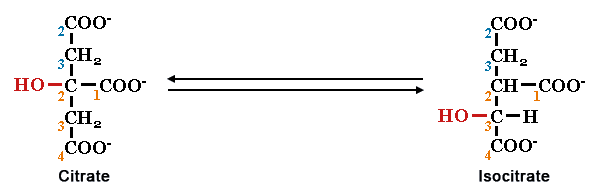
by Simon Fraser University
Even though it looks like individual functional groups are being oxidized or reduced, the total number of carbon, hydrogen and oxygen atoms in the molecule does not change. Prove it to yourself and count the numbers of each atom!
 Why is shape important in Biology?
Why is shape important in Biology?
Shape 14. Would you expect the substrate and product of an isomerization reaction to have the same shape? Justify your answer.
Types of Biological Reactions
As we look at some of the more common reactions in Biology it’s often easier to just think of them as small changes. For example, four amino acids can be converted from one type to another by changing just one or two functional groups. You can see these changes here:
Reactions
There are many different types of reactions that go on in cells. Our purpose here is to identify and describe the most common biochemical reactions.
 Biochemical Reactions Notes
Biochemical Reactions Notes
As you explore each of the different pairs of types of reactions, take note of how the functional groups are changing. Look for similarities and differences between the pair of changes. Record this using the template for a comparative contrastive map. For example, if we wanted to compare physical changes with chemical changes, we could compare and contrast them like this:
Common Biochemical Reactions
- Dehydration Synthesis and Hydrolysis
- Phosphorylation and Hydrolysis
- Phosphorylation and Decarboxylation
- Oxidation and Reduction
Dehydration Synthesis and Hydrolysis
This first pair of reactions we have already seen in Unit 1, so let's review by working through some interactives.
- Overview - Go to the last interactive: Building and Breaking Biomolecules: Launch Activity.
- Carbohydrates - Go to the third interactive: Building and Breaking Carbohydrates: Launch Activity
- Proteins - Go to the second interactive: Building and Breaking Proteins: Launch Activity
- Lipids - Go to the third interactive: Building and Breaking Fats: Launch Activity.
- Nucleic Acids - Go to the second interactive: Joining Nucleotides: Launch Activity.
Learn about the other common biochemical reactions in the following interactive.
Reactions2
 Biochemical Reactions
Biochemical Reactions
Using good details, compare and contrast the pairs of different biochemical reactions. Create your own comparing and contrasting map similar to the one below to show your understanding.
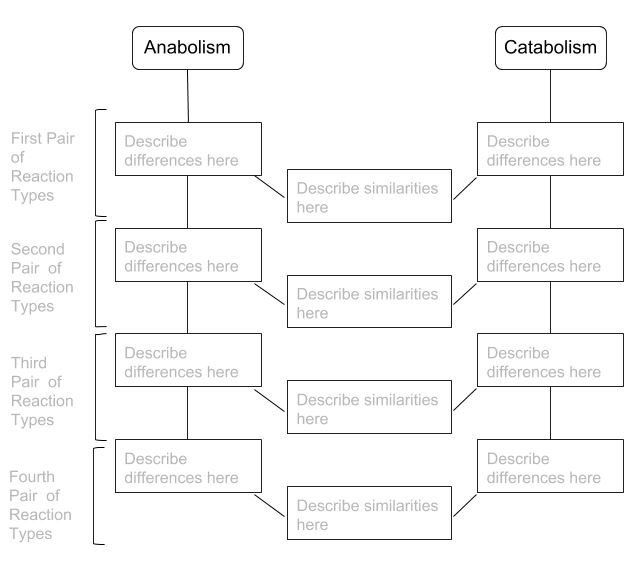
Remember that your comparisons and contrasts should:
- be meaningful;
- be well-organized and easy-to-follow;
- show your understanding of the vocabulary.
 How does energy move in food systems?
How does energy move in food systems?
Energy 4. Describe why it is necessary and efficient to recycle the ADP and inorganic phosphate rather that just release them as waste products.
CONSOLIDATION
Summary
- biochemical reactions involve energy changes;
- biochemical reactions can be grouped based on the type of change to the biological molecules or functional groups.